A Critical Review on the Role of Mycorrhizal Fungi in the Uptake of Phosphorus by Plants
- Review paper
- Open Access
- Published:
Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi
Register of Forest Science book 68,pages 33–43 (2011)Cite this commodity
Abstract
• Introduction
Phosphorus (P) is oftentimes the first or second element limiting aboveground net main productivity of forests. Besides low available inorganic orthophosphate (Pi) concentrations, soil may comprise high total P contents, as insoluble mineral P or as organic P. Most plants course mycorrhizal associations that amend their P nutrition. Iii main hypotheses have been proposed to explain this positive effect through an increase of (i) P mobilisation from mineral P, (2) P mobilisation from organic P and (3) soil exploration and P uptake. However, the positive outcome of mycorrhizal symbiosis may exist variable with the fungal species forming the association. This could be due to the different abilities of mycorrhizal fungi to mobilise P and/or to take up Pi from the soil.
• Objectives
The aim of this review was to examine our electric current knowledge nearly the capacity of ectomycorrhizal fungi to release organic compounds as depression-molecular-weight organic anions and phosphatases idea to have a role for mineral and organic P mobilisation, respectively. The diversity of Pi transporters among mycorrhizal species is also examined.
• Results
The primary conclusion is that the study of the functional diversity of ectomycorrhizal fungi in situ is still a challenging question and could exist addressed by combining different tools now available to make large-scale studies.
Introduction
Phosphorus (P) is an essential element for establish diet and tin only be taken up equally inorganic orthophosphate (Pi), either as H2PO − 4 or HPO 2− 4 , depending on the pH of soil solution. It has critical functions in many processes, such every bit free energy metabolism, synthesis of nucleic acids and membranes, as well every bit in photosynthesis (Raghothama 1999; Vance et al. 2003). In soil, free concentrations of Pi are thought to range from 1 to 10 μM (Bieleski 1973; Hinsinger 2001; Vance et al. 2003), and this low availability limits the productivity of plants in many terrestrial ecosystems. Information technology is ofttimes the first or second element limiting aboveground net primary productivity of forests. As well these low concentrations in complimentary Pi, soil may contain high levels of phosphorus that are non directly bachelor to plant roots or microorganisms as P is combined either to cations to form mineral P or to carbon-containing compounds to grade organic P. Given the Pi supply constraints in many soils, it is not surprising that plants take evolved strategies to learn and/or efficiently use P. Found adaptations that enhance the acquisition of inorganic Pi accept been reviewed many times (eastward.thousand. Bucher 2007; Lambers et al. 2008; Lynch and Brown 2008; Raghothama 1999; Richardson et al. 2009; Vance et al. 2003). Such adaptations include modifications to root structure and morphology, as well every bit biochemical (east.m. root exudates). However, in natural conditions, approximately fourscore% of country plants live in association with specialised soil fungi to course mycorrhizal roots. The most widespread mycorrhizal association exists between herbaceous plants and arbuscular mycorrhizal (AM) fungi forming AM symbiosis (Parniske 2008; Smith and Read 2008). Woody plants from the gymnosperms and several angiosperms growing in boreal and temperate regions too live in symbiotic association with mycorrhizal fungi that class ectomycorrhizal (ECM) roots (Marmeisse et al. 2004). Any the mycorrhizal blazon (AM or ECM), mycorrhizal fungi produce extraradical hyphae which are able to explore the soil away from the mycorrhizal roots and course a tight clan with the plant root where exchanges between fungal and root cells are occurring.
The germination of mycorrhizal roots is considered as the most widespread response to increase phosphate conquering by plants (Burleigh et al. 2002; Smith et al. 2000; Tibbett and Sanders 2002). This is mainly due to the repeated observation that mycorrhizal plants show growth improvement and increased found P content than non-mycorrhizal plants (Chalot et al. 2002; Smith and Read 2008). Three master hypotheses have been proposed to explicate this improved P diet of mycorrhizal plants: an increase of (1) P mobilisation from mineral P, (ii) P mobilisation from organic P and (3) soil exploration and P uptake. In particular, the office of extraradical hyphae is idea to be decisive for the functioning of the symbiosis (Finlay 2009). However, the positive effect of mycorrhizal symbiosis may be variable with the fungal species forming the association. This could be due to the different abilities of mycorrhizal fungi to mobilise P and/or to take up Pi from the soil. The aim of this review was to examine published information that may explain such differences between mycorrhizal fungal species, particularly those forming ectomycorrhizae.
Diversity of fungal species to mobilise mineral P
Soils comprise a large function of phosphate that can exist precipitated with calcium, atomic number 26 or aluminium to class many different potential phosphate minerals nether crystalline or amorphous course (Barber 1984). Some of the more common minerals are listed in Table i. In soils above pH 7, calcium phosphates should be dominant, whilst in acid soils, fe and aluminium phosphates are the dominant forms (Barber 1984). Solubility isotherms of phosphate minerals take been calculated equally a function of pH and concentration of Pi. Figure 1 shows some examples of common soil mineral phosphate isotherms calculated using a calcium action arbitrarily set up at pCa = ii.5, a value consequent with those found in non-calcareous soil solutions. The activities of Aliii+ and Fe3+ were controlled past the solubility of their oxides representing an average value of calcium and are given in Fig. 1. From these isotherms, it is possible to predict the solubility of each mineral when the pH and [H2PO4] in the soil solution are measured. If a point is to a higher place a line, the solution will exist supersaturated relative to that mineral. If a signal is under the line, the solution volition exist undersaturated (Barber 1984). In other words, Fig. 1 shows that crystalline aluminium and iron phosphate minerals will course at very depression [HtwoPOiv] at acidic pH (fifty-fifty at pH 5), whereas calcium phosphate minerals could be dissolved easily by a pH decrease from vii to five. In addition to precipitated forms, phosphate ions can be specifically adsorbed by soluble metal hydrous oxides or by clays (Hunt et al. 2007). Several organic compounds bearing carboxylic groups, either of high molecular weight such equally humic (HA) and fulvic acids (FA) or low-molecular-weight organic anions (LMWOAs) such as oxalate, may interact with Pi in soils to influence the amount of bachelor Pi in solution. Equally indicated by Hunt et al. (2007), the interactions betwixt organic compounds and mineral surfaces in soil may result in positive effects on Pi availability due to three processes—(ane) competition between carboxylates and Pi for mineral adsorption sites; (2) complexation of surface metals and release of these metals into solution, thereby removing adsorption sites; and (three) increased repulsion of phosphate anions by sorption of organic compounds to positive sorption sites—and in negative effects by enhancing the germination of cation bridges, leading to an increment in P sorption sites and a decrease in Pi availability. When assessed in solution, HA or FA purified from soils (Borggaard et al. 2005) or HA of commercial origin (Chase et al. 2007) displayed a low capacity to alter the adsorption of phosphate by metal oxides, leading to the conclusion that humic substances take limited influence on phosphate adsorption past aluminium and iron oxides (Borggaard et al. 2005; Guppy et al. 2005). In contrast to HA, oxalate supplied in the solution at low concentration (≈two or viii mM) together with goethite and gibbsite displayed a significant ability to decrease Pi adsorption on metallic oxides (Hunt et al. 2007). In this context, the product of LMWOAs by ectomycorrhizal fungi may play a decisive role for Pi release from phosphate minerals.
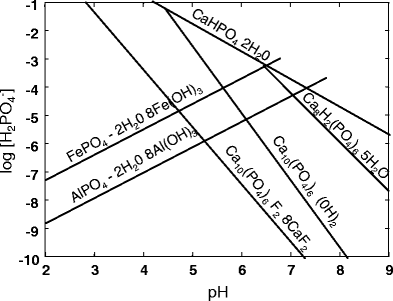
Solubility isotherms for indicated crystalline phases. Activity of calcium was arbitrarily set at pCa = 2.5. The activity of Al3+ and Fe3+ were controlled by the solubility of their oxides (redrawn from Fig. nine.3 in Barber 1984)
Ectomycorrhizal fungal species have been shown to be able to produce a range of LMWOAs (Plassard and Fransson 2009). The first studies on the capacity of ectomycorrhizal fungi to produce LMWOAs were carried out past Lapeyrie and colleagues (Lapeyrie 1988; Lapeyrie et al. 1987, 1991). Lapeyrie et al. (1991) used xi different ectomycorrhizal strains and found that oxalate production ranged from 27 to 120 μg mg−1 fungal dry weight, whereas other LMWOAs could not be detected. These results were confirmed by later experiments (Arvieu et al. 2003). In particular, Paxillus involutus, Suillus sp. and Rhizopogon roseolus were stiff oxalate producers (Arvieu et al. 2003; Lapeyrie et al. 1987). At present, approximately 30 species of ECM fungi belonging to the genera Cortinarius, Lactarius, Paxillus, Piloderma, Pisolithus and Suillus were plant to exist able to release substantial amounts of LMWOAs, with oxalate being the predominant class (Courty et al. 2010). Yet, a huge intraspecific variation in the capacity to release organic acids exists among these LMWOAs-producing fungal genera, as shown for P. involutus by Lapeyrie et al. (1991). On the other hand, almost no production of organic acids was detected in some ECM species belonging to the genera Amanita, Cenococcum, Hebeloma, Thelephora and Tylospora (Courty et al. 2010) or in the species Laccaria bicolor (Lapeyrie et al. 1991) or Hebeloma cylindrosporum (Arvieu et al. 2003). Taken together, these information betoken that a huge diversity of the power to produce oxalate occurs amongst ectomycorrhizal species and fifty-fifty among isolates of the aforementioned species. The ecological meaning of the diversity has not even so been established. In not-oxalate-producing species or isolates, whether this is due to a low production rate (through a lack or a low level of enzymes responsible for oxalate biosynthesis) or to the degradation of produced oxalate (past enzyme decarboxylation of oxalate) remains to be determined.
Also the specific diversity displayed by ectomycorrhizal fungal species, several environmental factors may increase or decrease organic anion production past ectomycorrhizal fungi. Exposure of ectomycorrhizal fungi to dissimilar metals (including Al, Fe, Lead, Cd, Cu and Ni) induced variable effects ranging from negative ones to positive ones (run into Table 1 in Plassard and Fransson 2009), with an exudation of oxalate increased by 15–45% later Pb and Cd exposure. However, exposure to Al and Iron by and large did not stimulate oxalate production by the fungal species used. In dissimilarity to metallic exposure, nitrogen source appears to be a major gene as in vitro studies carried out in pure culture bear witness a consistent stimulation of oxalic acid production in the presence of nitrate and inhibition by ammonium for P. involutus (Gharieb and Gadd 1999; Lapeyrie et al. 1987; 1991). We can hypothesise that it is the NH + 4 ion per se which has an inhibitory event on oxalate synthesis as organic nitrogen did not inhibit organic acrid release in R. roseolus, contrary to ammonium (Plassard, unpublished data). When nitrate is supplied equally the sole nitrogen source in the medium, the presence of calcium and bicarbonate ions was shown also to promote oxalate production in the fungus grown in pure culture (Lapeyrie et al. 1987) or in clan with the establish (Casarin et al. 2003, 2004).
From a physiological point of view, the efflux of oxalate through fungal prison cell membranes occurs equally anion transport because the cytosolic pH (around vii) is higher than the pK of oxalic acid (pChiliad for oxalate−/oxalate2− is 4.xix; Jones 1998), pregnant that the organic acid is actually nowadays as organic anion in the cytosol. Therefore, when carboxylates are exuded as anions, their charge could be balanced by a cation efflux that can be either One thousand+ or H+ (Roelofs et al. 2001) or alternatively by anion influx (Arvieu et al. 2003). Oxalate released with K+ or against anion influx will take simply complexing effects, whereas oxalate released with H+ volition have both complexing and acidifying effects. The production of oxalate only should be a meliorate strategy to mobilise Pi combined to metals in acidic soils, equally observed in Proteacae developing in highly weathered, acidic Australian soils and releasing citrate and Chiliad+ (Roelofs et al. 2001; Lambers et al. 2008). Conversely, the production of oxalate together with protons should exist a better strategy when Pi is combined with calcium (see Fig. 1). Interestingly, inter- and intraspecific differences in acidifying backdrop of oxalate-producing ectomycorrhizal species have been reported in vitro (Arvieu et al. 2003). Withal, in the study of Casarin et al. (2003, 2004), only the acidifying ECM species R. roseolus and the poor oxalate producer ECM species H. cylindrosporum were used in clan with Pinus pinaster plants grown in rhizoboxes containing a Mediterranean soil layer with a depression level of easily bachelor P. In these atmospheric condition, merely R. roseolus hyphae were able to release oxalate into the soil that combined with calcium to form calcium oxalate (Casarin et al. 2003). Oxalate release was accompanied by acidification and increase of Pi availability of rhizosphere soil (Casarin et al. 2004). Finally, this fungal association significantly increased the P nutrition of the host plant, indicating its ability to enhance Pi bioavailability from this soil. Information technology would be of great interest to use two contrasting ectomycorrhizal species, able to produce high amounts of oxalate with or without acidifying effect, to study the effects of complexing/acidifying properties due to organic anion release on P bioavailability in tropical acidic soils. Indeed, these soils are characterised by a high level of metal oxides where acidification could decrease P availability.
And so far, well-nigh of our knowledge virtually the capacities of ECM species to produce organic anions comes from in vitro studies (Rosling 2009). Despite the fact that in vitro experiments are valuable tools to study processes, systematic in situ measurement of oxalate release by ectomycorrhizal tips could bring more relevant data on the ecological point of view. Progress in this field was hampered by the lack of an easy method to measure LMWOAs in field-sampled ectomycorrhizal tips. Recently, Rineau et al. (2008) reported a microplate assay making it possible to measure the release of oxalate at the level of individual ectomycorrhizal tips. The apply of this method could be a very valuable tool to appraise at least the capacities of oxalate released by ectomycorrhizal species even if they are non grown in vitro. In addition, the comparison of oxalate release capacity by these ectomycorrhizal tips with the actual concentration of oxalate in soil sample surrounding these tips could help quantify the fate of LMWOAs released into the mycorhizosphere. However, determining if oxalate—or other LMWOAs—are released with or without protons in situ remains a challenging issue.
Multifariousness of fungal species to mobilise organic P
Remarkably, also the low level of available gratuitous Pi, soils contain a loftier amount of P that is linked to C-containing compounds to class organic P (Po). The majority of the organic phosphorus is nowadays as phosphate esters (C–O–P bonds) either in the class of phosphate monoesters, including inositol phosphates, or phosphate diesters, such as nucleic acids and phospholipids, together with small quantities of phosphonates (C–P bonds; Condron et al. 2005; Magid et al. 1996). To be used by plants and soil microorganisms, the phosphate grouping of Po compounds must be released from the ester bond linking it to carbon by enzymes that are phosphatases. Depending on their substrate, the enzymes can exist phosphomonoesterases or phosphodiesterases. Most of the studies addressing the release of phosphatases use artificial phosphomonesters such as p-nitrophenyl phosphate (pNPP) based on the procedure described in Tabatabai and Bremner (1969) or the fluorescent assay based on the release of 4-methylumbelliferone from 4-methylumbelliferone-phosphate (MUP; Courty et al. 2005, 2006; Pritsch et al. 2004; Pritsch and Garbaye 2011) to guess phosphomonoesterase action.
Depending on the pH of the incubation medium, one tin distinguish acid phosphomonoesterase action (ACP) from element of group i phosphomonoesterase activity (ALP), measured respectively at pH around v (ACP) and 8 or more (ALP; Bae and Barton 1989; van Aarle and Plassard 2010). Even so, so far, most of the ECM fungi showed maximal activities of released or surface-leap phosphomonoesterase at acidic pH when assayed with pNPP (Tibbett 2002) or with MUP (Courty et al. 2005). ALP was measured in cell extracts of Cenococcum graniforme (renamed Cenococcum geophilum), and although the enzyme showed a loftier association with prison cell wall material, information technology was not released into the external medium from immature fungal cultures (Bae and Barton 1989). Indeed, Antibus et al. (1986) did non measure out a significant surface ALP in the same species but rather an ACP. It tin can be hypothesised that intracellular ALP volition exist involved in internal recycling of fungal organic P, whereas surface-leap or released ACP will be involved in the mineralization of soil organic P. Indeed, every bit about woods soils are non alkaline, the production of acrid phosphatases past ECM fungi seems to exist relevant of the field conditions encountered by ECM fungi.
Different abilities to release ACP were reported among ectomycorrhizal species and strains when grown in pure culture (eastward.g. Matumoto-Pintro 1996 in Quiquampoix and Mousain 2005; Tibbett et al. 1998; Nygren and Rosling 2009). For example, the measurement of pNPPase activities in ten strains of Basidiomycetes (Laccaria laccata, Suillus collinitus (three strains), Suillus granulatus, Suillus luteus, H. cylindrosporum (two strains), P. involutus and Rhizopogon rubescens) after civilization of the mycelia in a depression-phosphate medium showed that but six of them had free extracellular pNPPase action (one strain of S. collinitus, S. granulatus, S. luteus; both strains of H. cylindrosporum and R. rubescens). In add-on, the two H. cylindrosporum strains presented exceptional high level of ACP activities they secreted into the external medium (Quiquampoix and Mousain 2005). The power of Hebeloma species to increase the proportion of extracellular pNPPase compared to the surface-bound action in the case of Pi depletion was also reported by Tibbett et al. (1998). The loftier ability to release acid phosphatases into the external medium was recently confirmed in another isolate of H. cylindrosporum by Louche et al. (2010). In this study, four fractions containing ACPase activity were separated from the culture medium, suggesting that this fungal species could be able to produce several isoforms of acrid phosphatase. Each fraction was able to hydrolyse a range of phosphomonoesters, merely the characteristics of the corresponding proteins remain to be determined. From a molecular betoken of view, a cistron coding for an acid phosphatase (XP 001887867, http://www.ncbi.nlm.nih.gov/) is available from the genome of Fifty. bicolor recently completely sequenced (Martin et al. 2008). The predicted poly peptide is very close to the acrid phosphatase AfPhoA (Q8X176) characterised in Aspergillus fumigatus (Bernard et al. 2002) as well as to 2 other acrid phosphatases identified in the basidiomycete Pholiota nameko (PNAP1: BAD00139 and PNAP2: BAD00140; Fig. two). Interestingly, the different polypeptides share three domains that are highly conserved among these acid phosphatases (Bernard et al. 2002; Fig. ii). However, no specific functions have been attributed nonetheless to these conserved domains. Whether acid phosphatase fractions separated from the culture medium of H. cylindrosporum stand for to one cistron homologous to PhoA or to iv dissimilar genes remains to be determined.

Comparing of the predicted amino acrid sequences of four acid phosphatases identified in the ectomycorrhizal L. bicolor, LbPhoA (accession XP 001887867); the ascomycete A. fumigatus, AfPhoA (accession Q8X176) and in the saprotroph basidiomycete P. nameko, PNAP1 (accession BAD00139) and PNAP2 (accession BAD00140). The sequences were retrieved from NCBI (http://www.ncbi.nlm.nih.gov/). Identical residues are indicated by solid boxes; similar residues are indicated by grey boxes. The lines indicate highly conserved motifs among the proteins
Amidst the different forms of organic P extracted from soil, phytate, corresponding to the salt of myo-inositol hexakisphosphate (IP6), is oft the dominant form of organic P (Turner et al. 2002). To be used as a P source, orthophosphate groups linked to the inositol ring with an ester bond must exist released by the action of phosphatases named phytases. Saprotrobic fungi are able to release these enzymes that belong to the grade of histidine acid phytase (HAPhy) that can be six-phytase (EC 3.1.3.26) or 3-phytase (EC 3.1.3.eight), depending on the position of the first phosphate group released by the enzyme (Mullaney and Ullah 2007). Alignment of available amino acid sequences of HAPhys show that the N-terminal motif RHGXRXP and the C-last motif HD that etch the active site are the only ones conserved among the HAPhy identified in prokaryotes or eukaryotes (Mullaney and Ullah 2007).
These enzymes are acrid phosphatases and have been shown to release up to five Pi and inositol monophosphate per inositol hexakisphosphate. Experimentally, phytase activeness measurement is based on the release of Pi from phytate salt supplied in incubation medium because there is no bogus substrate every bit convenient as pNPP or MUP. However, the sensitivity of Pi release from phytate hydrolysis is higher when the green malachite method is used (Ohno and Zibilske 1991) as the minimal Pi concentration that could be detected with this method is around ii μM. In ectomycorrhizal fungi, phytase activity has been measured in the ten ectomycorrhizal isolates cited above (Matumoto-Pintro 1996 in Quiquampoix and Mousain 2005). Only four isolates had free extracellular phytase activity (one strain of Southward. collinitus, S. granulatus, and both strains of H. cylindrosporum; Quiquampoix and Mousain 2005). Yet, the rate of Pi release was low compared to that measured for para-nitrophenol release, with values ranging from ane% to eight% in the four fractions separated from the culture medium of H. cylindrosporum (Louche et al. 2010).
When measured in vitro, an inverse relationship has by and large been reported between Pi concentration in culture media and ACP, indicating that ACP is derepressed by Pi starvation (Tibbett 2002). Higher values of surface-spring ACP take too been measured in ectomycorrhizal roots (see, e.g. Ali et al. 2009; van Aarle and Plassard 2010) and hyphae (van Aarle and Plassard 2010) belonging to ectomycorrhizal plants grown in soil with a low level of available P, confirming the results obtained in vitro. In contrast, it seems that the presence of organic P did non activate the production of ACP in ECM fungi every bit no differences in enzyme production were found after growth on inorganic and organic P sources (Antibus et al. 1992; Nygren and Rosling 2009). The release of extracellular enzymes is costly and ane tin imagine that this strategy—synthesis and release of ACP only in low P availability—will avoid a waste of free energy for the fungus when Pi is available in the external solution. However, in improver to Pi availability, N fertilisation applied in the field appears also as an important factor to regulate ACP activity, as shown by a recent study made past Taniguchi et al. (2008). The authors used seven ECM fungi to inoculate Pinus thunbergii seedlings in control atmospheric condition: C. geophilum, Rhizopogon sp., South. granulatus, Tomentella sp. one, Tomentella sp. ii, Amanita sp. and 1 unidentified ECM fungus T01. Once inoculated, the seedlings were cultivated in sterilised soil, whether or not supplemented with N supplied as ammonium nitrate. ACP was measured using pNPP, and the results showed that in non-fertilised conditions (command group), the activeness was significantly higher in Tomentella sp. 1, Tomentella sp. two or Amanita sp. than in S. granulatus or Rhizopogon sp., unidentified ECM fungus T01 and in the non-mycorrhizal root tips. In fertilised conditions (N grouping), activity of root tips was significantly higher in the seedlings inoculated with Tomentella sp. 1 or Tomentella sp. 2 than in the seedlings inoculated with Southward. granulatus or Rhizopogon sp. and in the non-inoculated seedlings. The awarding of N significantly increased the activity measured in NM and South. granulatus root tips. This increase of p-PNPase activeness was attributed to the depletion of Pi due to better P uptake and accumulation in plant or fungal tissues following the enhanced Northward availability (Taniguchi et al. 2008).
Despite the fact that ECM fungi are able to release ACPase, especially when Pi availability is low, the efficiency of these enzymes to mobilise Pi from organic P in forest soils is still a matter of debate. As an case, studies using ectomycorrhizal roots and their external mycelium reported opposite conclusions regarding the use of inositol phosphate P by the mycorrhizal fungus (Antibus et al. 1997; Colpaert et al. 1997). These experiments were carried out in simplified conditions (perlite as growing medium and solution containing 32P-labelled inositol phosphate, respectively) that overestimate the actual result of ECM fungi as the availability of the substrate is not limiting. Indeed, information technology is important to keep in mind that the studies measuring P accumulation in mycorrhizal plants grown in substrate containing added soluble organic P compounds may overestimate the actual function of ECM fungi to use organic P in field conditions. This is particularly important in the case of inositol phosphate, a polyanion that can be hands combined with mineral surfaces (atomic number 26 and aluminium oxides, calcium) and thus made unavailable to phytases (Giaveno et al. 2010). Nevertheless, the ability to hydrolyse a large spectrum of phosphomonoesters, every bit shown by Louche et al. (2010), may exist an advantage in soil weather. The phosphatases could also be involved in recapturing of excreted constitute or fungal compounds (Barrett-Lennard et al. 1993) or breaking down and recycling the phospholipids from former hyphae (Nygren and Rosling 2009), avoiding therefore the loss of P into the surround.
Diversity in P uptake
Considerable increased soil exploration past mycorrhizal fungi is thought to play a major part in overcoming the Pi depletion zone occurring around the roots. As an example, data obtained in young pot-grown Pinus taeda showed that the absorbing surface contributed by the hyphae of the ectomycorrhizal species Pisolithus tinctorius represented about 75% of the uptake potential absorbing area and over 99% of the absorbing length of the whole root system (Rousseau et al. 1994). Inoculation of P. taeda plants with C. geophilum was by far less efficient to improve P nutrition of the host plant. This was attributed to a much lower hyphal growth of C. geophilum (2.80 m g−ane soil) compared with that measured in P. tinctorius (6.78 m g−1 soil), in combination with the absence of rhizomorphs formed by C. geophilum (Rousseau et al. 1994). These data illustrate the importance of hyphal growth and rhizomorph formation on P uptake and its net transfer to the ectomycorrhizal plant. Notwithstanding, information technology should be noticed that ectomycorrhizal species tin display different patterns to explore the soil according to the classification proposed past Agerer (2001). Some examples of species belonging to different exploration types are given in Table 2 (Hobbie and Agerer 2010). In theory, species belonging to "long-exploration" type or to "medium-distance" should ensure a better soil exploration than the species of the "contact" blazon. However, determining the relationships between hyphal density, exploration type and the actual efficiency of ECM species to amend P uptake of trees in natural weather is still a challenging issue.
From a functional point of view, studies carried out with intact plants grown in microcosms showed that extraradical hyphae and mycelial strands of Suillus bovinus interconnecting Pinus contorta and Pinus sylvestris plants were besides able to accept up 32P-Pi and to translocate labelled P to the shoots (Finlay and Read 1986). This transport of P is unidirectional (from the fungal to the host cells) as no translocation of 32P supplied to non-mycorrhizal roots was detected in Due south. bovinus mycelium (Finlay and Read 1986). External hyphae associated with young P. sylvestris grown in perlite strongly enhanced the Pi uptake chapters of the pine root arrangement by decreasing the K m and increasing V max of Pi uptake rates in ECM plants compared with non-mycorrhizal plants (Van Tichelen and Colpaert 2000). However, plants associated with P. involutus were more efficient to take up Pi than those associated with Southward. bovinus and Thelephora terrestris (Table 3), suggesting that the Pi transporters may exhibit unlike properties amongst the associated fungal species.
Regarding the identification of phosphate transporters in fungi, ii types of high-affinity Pi transporters have been characterised, which are either Pi:H+ or Pi:Na+ transporters. In yeast, these 2 transporters are encoded by two genes, PHO84 (Bun-ya et al. 1991) and PHO89 (Martinez and Persson 1998), whose expression is activated when the cells encounter a limitation in external Pi (Persson et al. 2003). The PHO84 ship system displayed K chiliad values for external Pi ranging from i to 15 μM, whereas PHO89 displayed a K m for external Pi of 0.5 μM (Persson et al. 2003). In mycorrhizal fungi, the starting time data regarding the identification of phosphate transporters possibly involved in Pi uptake were obtained in the AM species Glomus versiforme associated with Medicago truncatula (Harrison and van Buuren 1995). Pi uptake mediated past the transporter (named GvPT) in yeast is dependent on external pH, suggesting that it is operating via a proton-coupled symport and exhibited an apparent K m value of 18 μM Pi (Harrison and van Buuren 1995). Furthermore, one partial cDNA (GmosPT) and 1 full-length cDNA (GiPT) putatively coding for Pi transporters accept been identified in two AM species, Glomus mosseae and Glomus intraradices, respectively (Benedetto et al. 2005; Maldonado-Mendoza et al. 2001). Although the polypeptides encoded past GmosPT and GiPT were not functionally characterised yet, they all clustered with fungal Pi:H+ polypeptides (Tatry et al. 2009). All these transcripts accept been predominantly detected in extraradical hyphae, with their expression level enhanced past low P availability, such as reported in Chiliad. intraradices (Maldonado-Mendoza et al. 2001; Olsson et al. 2006) and G. mosseae (Benedetto et al. 2005). Taken as a whole, these information advise a role in Pi acquisition from the soil for all these Pi transporters despite the rather loftier value of K m measured for Pi uptake mediated by GvPT expressed in yeast.
Regarding ECM fungi, data are available from three species: two Basidiomycetes, L. bicolor and H. cylindrosporum, and one Ascomycete, Tuber melanosporum. In Fifty. bicolor, whose the genome has been completely sequenced, v genes possibly coding for Pi transporters belonging to the Mafor Facilitating Superfamily (MFS) of transporters accept been identified and named LbPht1;1 to LbPht1;5 (http://genome.jgi-psf.org/Lacbi1/Lacbi1.home.html). Ii other genes, HcPT1 and HcPT2, have been identified in H. cylindrosporum (Tatry et al. 2009). The peptide sequence of HcPT1 is very shut to two Fifty. bicolor predicted polypeptides (LbPht1;4 and LbPht1;5) and HcPT2 is close to the iii other ones (LbPht1;1–3). As in AM fungi, the seven polypeptides cluster fungal Pi:H+ transporters. Notwithstanding, the genome of T. melanosporum (http://www.genoscope.cns.fr/externe/GenomeBrowser/Tuber/) contains at least three genes coding for Pi transporters. Two of them encode putative Pi:H+ transporters belonging to the MFS, homologous to PHO84 (high analogousness) and to PHO87 (depression affinity) in yeast. The final gene should encode a poly peptide belonging to some other transporter family, the Inorganic Phosphate Transporter (PiT) family, very shut to the high-affinity Pi:Na+ polypeptide NcPHO4 characterised in the mould Ascomycete Neurospora crassa.
The polypeptides encoded by the genes identified in 50. bicolor or T. melanosporum have not been characterised however. Nevertheless, this was done for HcPT1 and HcPT2 (Tatry et al. 2009) which were shown able to mediate Pi:H+ symport with different affinities for Pi, the Thousand m values being 55 and 4 μM, respectively, for HcPT1 and HcPT2. The credible Thou m of HcPT2 was therefore comparable to that reported for PHO84 and lower than that of the factor GvPT (xviii μM), which is the only value for a Pi transporter of AM fungi currently available for comparison (Harrison and van Buuren 1995). The value of One thousand m institute for HcPT2 was in the same range as those found in intact plants of P. sylvestris (see Table three), suggesting that this phosphate transporter could play a great part in Pi uptake into fungal cells. Expression levels of HcPT1 and HcPT2 were quantified as a function of external Pi availability in the hyphae grown in pure culture or associated with their host found P. pinaster. Levels of HcPT1 transcripts were always higher in fungal cells exposed to Pi starvation in solution or to low Pi availability in soil, suggesting that the regulation of this transport system is shut to that of PHO84 in yeast. In contrast, transcript levels of HcPT2 were less dependent on Pi availability in fungal cells grown in vitro and were upregulated in ectomycorrhizal roots grown in soil with loftier P availability (Tatry et al. 2009). Taken as a whole, these results indicate that H. cylindrosporum might employ HcPT1 to mediate Pi uptake when soil P availability is low and HcPT2 when soil P availability is high. It is therefore intriguing to find such rather high apparent affinity value measured for HcPT1 expressed in yeast and its functioning when Pi availability is depression. Every bit proposed past Tatry et al. (2009), this could exist due to heterologous expression that may modify the actual rates of Pi uptake and specially the apparent K chiliad value. Indeed, one would look a low value of credible 1000 m of Pi uptake in ectomycorrhizal fungi (see Table 3), shut to that found in HcPT2. It should be noticed that the heterologous expression of HcPT1 required starving the yeasts for Pi (Tatry et al. 2009), a feature that was not observed for HcPT2. These data suggest that HcPT1 expression may have been significantly modified by the yeast mechanism, contrary to HcPT2, leading to the overestimated credible Grand one thousand value. In this context, information technology will be very interesting to carry out the functional label of Pi transporters identified in other ectomycorrhizal fungi, particularly those shut to HcPT1. These information will be helpful to go a amend quantification of the actual capacities of Pi send by these proteins. Also, the functional label of the polypeptides encoded by the genes discovered in T. melanosporum genome will ostend whether this fungus is able to take up Pi in alkaline weather condition, where protons are not available.
Concluding remarks
From this review, information technology is clear that mycorrhizal fungi exhibit a large diversity in their ability to mobilise mineral or organic P from the soil, also equally in their efficiency to explore the soil and to accept upward Pi from the soil solution. It is clear likewise that nearly of the data accept been obtained by carrying studies on a small number of fungal species. The challenge volition be now to notice tools to extend our studies in situ to increase our comprehension of the functioning of wood ecosystems. Regarding insoluble mineral P mobilisation, it seems that the release of LMWOAs such equally oxalate is of great importance to enhance P availability. However, we need to know better what the diversity is and how this production of oxalate is regulated by environmental factors such as nitrogen source. There is no molecular tool available however, but measurement of oxalate production capacities past ectomycorrhizal tips (Rineau et al. 2008) in various environmental weather, together with the determination of available sources of Northward (that could be identified by incubating field soil samples in laboratory conditions), should help us improve our knowledge of this regulation in the field. However, despite the importance of the accompanying cation on the actual efficiency of LMWOA release, determining whether oxalate—or other LMWOAs—are released with or without protons in situ remains a challenging issue.
Regarding the mobilisation of organic P, the all-encompassing use of microplate assay (Pritsch et al. 2004; Courty et al.; 2005; Pritsch and Garbaye 2011, this issue) for measuring acid phosphatase activities on individual root tips, together with the molecular identification of the fungal species forming the ectomycorrhizal tip, should be a valuable tool to extend our knowledge about the factors that regulate the abundance of phosphatases released into the soil. Alternatively, molecular tools could be designed to written report the diversity of these enzymes in the field. Finally, regarding soil exploration and Pi uptake efficiency, the variety of Pi transporters among ectomycorrhizal species is even so a challenging question in the field. Information technology should be useful to combine studies dealing with exploration types and variability of Pi transporters that could be assessed using molecular tools.
References
-
Agerer R (2001) Exploration types of ectomycorrhizae. A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107–114
-
Ali MA, Louche J, Legname E, Duchemin M, Plassard C (2009) Pinus pinaster seedlings and their fungal symbionts bear witness high plasticity in phosphorus acquisition in acidic soils. Tree Physiol 29:1587–1597
-
Antibus RK, Kroehler CJ, Linkins AE (1986) The effects of external pH, temperature and substrate concentration on acrid phosphatase activity of ectomycorrhizal fungi. Can J Bot 64:2383–2387
-
Antibus RK, Kroehler CJ, Linkins AE (1992) Phosphatase activities and phosphorus uptake from inositol phosphate by ectomycorrhizal fungi. Tin J Bot 70:794–801
-
Antibus RK, Bower D, Dighton J (1997) Root surface phosphatase activities and uptake of 32P-labelled inositol phosphate in field-collected gray birch and blood-red maple roots. Mycorrhiza 7:39–46
-
Arvieu J-C, Leprince F, Plassard C (2003) Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Ann For Sci 60:815–821
-
Bae KS, Barton LL (1989) Alkaline phosphatase and other hydrolases produced by Cenococcum graniforme, an ectomycorrhizal fungus. Appl Environ Microbiol 55:2511–2516
-
Hairdresser S (1984) Soil nutrient bioavailability. A mechanistic approach. Wiley, New York, 398 pp
-
Barrett-Lennard EG, Dracup M, Greenway H (1993) Role of extracellular phosphatases in the phosphorus diet of clover. J Exp Bot 44:1595–1600
-
Benedetto A, Magurno F, Bonfante P, Lanfranco L (2005) Expression profiles of a phosphate transporter cistron (GmosPT) from the endomycorrhizal fungus Glomus mossae. Mycorrhiza xv:620–627
-
Bernard Grand, Mouyna I, Dubreucq G, Debeaupuis J-P, Fontaine T, Vorgias C, Fuglsang C, Latge J-P (2002) Characterization of a cell wall acid phosphatase (PhoAp) in Aspergillus fumigatus. Microbiology 148:2819–2829
-
Bieleski RL (1973) Phosphate pools, phosphate transport and phosphate availablility. Ann Rev Plant Physiol 24:225–252
-
Borggaard OK, Raben-Lange B, Gimsing AL, Strobel BW (2005) Influence of humic substances on phosphate adsorption by aluminum and iron oxides. Geoderma 127:270–279
-
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhizal interfaces. New Phytol 173:xi–26
-
Bun-ya Northward, Nishimura M, Harashima S, Oshima Y (1991) The PHO84 cistron of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Jail cell Biol eleven:3229–3238
-
Burleigh SH, Cavagnaro T, Jakobsen I (2002) Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J Exp Bot 53:1593–1601
-
Casarin 5, Plassard C, Souche G, Arvieu J-C (2003) Quantification of oxalate ions and protons released by ectomycorrhizal fungi in rhizosphere soil. Agronomie 23:461–469
-
Casarin 5, Plassard C, Hinsinger P, Arvieu J-C (2004) Quantification of ectomycorrhizal furnishings on the bioavailability and mobilization of soil P in the rhizosphere of Pinus pinaster. New Phytol 163:177–195
-
Chalot Yard, Javelle A, Blaudez D, Lambilliote R, Cooke R, Sentenac H, Wipf D, Botton B (2002) An update on nutrient transport processes in ectomycorrhizas. Establish Soil 244:165–175
-
Colpaert JV, van Laere A, van Tichelen KK, van Assche JA (1997) The utilise of inositol hexaphosphate every bit a phosphorus source past mycorrhizal and not-mycorrhizal Scots pino (Pinus sylvestris). Funct Ecol 11:407–415
-
Condron LM, Turner BL, Cade-Menun BJ (2005) The chemistry and dynamics of soil organic phosphorus. In: Sims JT, Sharpley AN (eds) Phosphorus: agronomics and the environment. ASA-CSSA-SSSA, Madison, pp 87–121
-
Courty P-E, Pritsch K, Scholter M, Hartman A, Garbaye J (2005) Activeness profiling of ectomycorrhiza communities in 2 forest soils using multiple enzymatic tests. New Phytol 167:309–319
-
Courty P-E, Pouysegur R, Buée M, Garbaye J (2006) Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biol Biochem 38:1219–1222
-
Courty P-Due east, Buée M, Diedhou AG, Frey-Klett P, Le Tacon F, Rineau F, Turpault Thousand-P, Uroz S, Garbaye J (2010) The function of ectomycorrhizal communities in wood ecosystem processes: new perspectives and emerging concepts. Soil Biol Biochem 42:679–698
-
Finlay R (2009) Ecological aspects of mycorrhizal symbiosis: with special accent on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot 59:1115–1126
-
Finlay R, Read DJ (1986) The structure and function of the vegetative mycelium of ectomycorrhizal plants. II. The uptake and distribution of phosphorus past mycelial strands interconnecting host plants. New Phytol 103:157–165
-
Gharieb MM, Gadd GM (1999) Influence of nitrogen source on the solubilization of natural gypsum (CaSO4 ⋅2H2O) and the formation of calcium oxalate past different oxalic and citric acid-producing fungi. Mycol Res 103:473–481
-
Giaveno C, Celi L, Richardson AE, Simpson RJ, Barberis E (2010) Interaction of phytases with minerals and availability of substrate impact the hydrolysis of inositol phosphates. Soil Biol Biochem 42:491–498
-
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Austr J Soil Res 43:189–202
-
Harrison MJ, van Buuren ML (1995) A phosphate transporter from the mycorrhizal mucus Glomus versiforme. Nature 378:626–629
-
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemic changes: a review. Plant Soil 237:173–195
-
Hobbie EA, Agerer R (2010) Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Institute Soil 327:71–83
-
Hunt JF, Ohno T, He Z, Honeycutt CW, Dail BD (2007) Inhibition of phosphorus sorption to goethite, gibbsite, and kaolin by fresh and decomposed organic thing. Biol Fertil Soils 44:277–288
-
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
-
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Found nutrient-conquering strategies change with soil historic period. Trends Ecol Evol 23:95–103
-
Lapeyrie F (1988) Oxalate synthesis from soil bicarbonate by the mycorrhizal fungus Paxillus involutus. Plant Soil 110:3–8
-
Lapeyrie F, Chilvers GA, Bhem CA (1987) Oxalic acid synthesis by the mycorrhizal fungus Paxillus involutus (Batsch. Ex Fr.) Fr. New Phytol 106:139–146
-
Lapeyrie F, Ranger J, Vairelles D (1991) Phosphate-solubilizing activity of ectomycorrhizal fungi in vitro. Can J Bot 69:342–346
-
Louche J, Ali MA, Cloutier-Hurteau B, Sauvage F-X, Quiquampoix H, Plassard C (2010) Efficiency of acrid phosphatases secreted from the ectomycorrhizal fungus Hebeloma cylindrosporum to hydrolyse organic phosphorus in podzols. FEMS Microbiol Ecol 73:323–335
-
Lynch JP, Brown KM (2008) Root strategies for phosphorus acquisition. In: White PJ, Hammond JP (eds) The ecosphysiology of plant–phosphorus interactions. Springer, Berlin, pp 83–96
-
Magid J, Tiessen H, Condron LM (1996) Dynamics of organic phosphorus in soils under natural and agricultural ecosystems. In: Piccolo A (ed) Humic substances in terrestrial ecosystems. Elsevier, Amsterdam, pp 429–466
-
Maldonado-Mendoza IE, Dewbre GR, Harrison MJ (2001) A phosphate transporter gene from the actress-radical mycelium of an arbuscular mycorrhizal mucus Glomus intraradices is regulated in response to phosphate in the environment. Mol Constitute Microb Interact 14:1140–1148
-
Marmeisse R, Guidot A, Gay G, Lambilliotte R, Sentenac H, Combier J-P, Melayah D, Fraissinet-Tachet L, Debaud J-C (2004) Hebeloma cylindrosporum—a model species to study ectomycorrhizal symbiosis from gene to ecosystem. New Phytol 163:481–498
-
Martin F, Aerts A, Ahrén D, Brun A, Danchin EGJ, Duchaussoy F, Gibon J, Kohler A, Lindquist Eastward, Pereda V, Salamov A, Shapiro HJ, Wuyts J, Blaudez D, Buée M, Brokstein P, Canbäck B, Cohen D, Courty PE, Coutinho PM, Delaruelle C, Detter JC, Deveau A, DiFazio S, Duplessis S, Fraissinet-Tachet Fifty, Lucic E, Frey-Klett P, Fourrey C, Feussner I, Gay G, Grimwood J, Hoegger PJ, Jain P, Kilaru South, Labbé J, Lin YC, Legué V, Le Tacon F, Marmeisse R, Melayah D, Montanini B, Muratet M, Nehls U, Niculita-Hirzel H, Oudot-Le Secq MP, Peter M, Quesneville H, Rajashekar B, Reich M, Rouhier N, Schmutz J, Yin T, Chalot M, Henrissat B, Kües U, Lucas Southward, Van de Peer Y, Podila GK, Polle A, Pukkila PJ, Richardson PM, Rouzé P, Sanders IR, Stajich JE, Tunlid A, Tuskan Yard, Grigoriev IV (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452:88–92
-
Martinez P, Persson B (1998) Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol Gen Genet 258:628–638
-
Mullaney EJ, Ullah AHJ (2007) Phytases: attributes, catalytic mechanisms and applications. In: Turner BL, Richardson AE, Mullaney EJ (eds) Inositol phosphates: linking agriculture and the environment. CAB International, Wallingford, pp 97–110
-
Nygren CMR, Rosling A (2009) Localisation of phosphomonoesterase activity in ectomycorrhizal fungi grown on different phosphorus sources. Mycorrhiza 19:197–204
-
Ohno T, Zibilske L (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895
-
Olsson PA, Hansson MC, Burleigh SH (2006) Effect of P availability on temporal dynamics of carbon allotment and Glomus intraradices high-analogousness P transporter factor induction in arbuscular mycorrhiza. Appl Environ Microbiol 72:4115–4120
-
Parniske M (2008) Arbuscular mycorrhiza: the female parent of plant root endosymbioses. Nat Rev Microbiol 6:763–775
-
Persson BL, Lagerstedt JO, Pratt JR, Pattison-Granberg J, Lundh K, Shokrollahzadeh S, Lundh F (2003) Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr Genet 43:225–244
-
Plassard C, Fransson P (2009) Regulation of low-molecular weight organic acrid product in fungi. Fungal Biol Rev 23:thirty–39
-
Pritsch M, Garbaye J (2011) Enzyme secretion past ECM-fungi and exploitation of mineral nutrients from soil organic matter. Ann For Sci 68. doi:10.1007/s13595-010-0004-8
-
Pritsch K, Raidl S, Marksteiner East, Blaschke H, Agerer R, Schloter M, Hartmann A (2004) A rapid and highly sensitive method for measuring enzyme activities in single mycorrhizal tips using 4-methylumbelliferone-labelled fluorogenic substrates in a microplate system. J Microb Meth 58:233–241
-
Quiquampoix H, Mousain D (2005) Enzymatic hydrolysis of organic phosphorus. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment. CAB International, Wallingford, pp 89–112
-
Raghothama KG (1999) Phosphate conquering. Ann Rev Plant Physiol Constitute Mol Biol 50:665–693
-
Richardson AE, Barea J-G, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion past microorganisms. Establish Soil 321:305–339
-
Rineau F, Courty P-Eastward, Uroz S, Buée M, Garbaye J (2008) Simple microplate assays to measure iron mobilization and oxalate secretion past ectomycorhizal tree roots. Soil Biol Biochem xl:2460–2463
-
Roelofs RFR, Rengel Z, Cawthray GR, Dixon KW, Lambers H (2001) Exudation of carboxylates in Australian Proteaceae: chemical limerick. Constitute Jail cell Environ 24:891–904
-
Rosling A (2009) Trees, mycorrhiza and minerals—field relevance of in vitro experiments. Geomicrobiol J 26:389–401
-
Rousseau JVD, Sylvia DM, Fox AJ (1994) Contribution of ectomycorrhiza to the potential nutriment-absorbing surface of pine. New Phytol 128:639–644
-
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, Amsterdam, 787 pp
-
Smith FW, Rae AL, Hawkesford MJ (2000) Molecular mechanisms of phosphate and sulphate transport in plants. Biochim Biophys Acta 1465:236–245
-
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activeness. Soil Biol Biochem 1:301–307
-
Taniguchi T, Kataoka R, Futai Chiliad (2008) Plant growth and nutrition in pine (Pinus thunbergii) seedlings and dehydrogenase and phosphatase activity of ectomycorrhizal root tips inoculated with seven private ectomycorrhizal fungal species at high and low nitrogen conditions. Soil Biol Biochem 40:1235–1243
-
Tatry M-Five, El KE, Lambilliotte R, Corratgé C, van Aarle I, Amenc LK, Alary R, Zimmermann S, Sentenac H, Plassard C (2009) Two differentially regulated phosphate transporters from the symbiotic mucus Hebeloma cylindrosporum and phosphorus conquering past ectomycorrhizal Pinus pinaster. Constitute J 57:1092–1102
-
Tibbett M (2002) Consideration on the utilise of the p-nitrophenyl phosphomonoesterase assay in the written report of the phosporus nutrition of soil borne fungi. Microbiol Res 157:221–231
-
Tibbett M, Sanders FE (2002) Ectomycorrhizal symbiosis can enhance plant nutrition through improved admission to discrete organic nutrient patches of high resource quality. Ann Bot 89:783–789
-
Tibbett M, Sanders Iron, Cairney JWG (1998) The effect of temperature and inorganic phosphorus supply on growth and acid phosphatase production in arctic and temperate strains of ectomycorrhizal Hebeloma spp. in axenic civilisation. Mycol Res 102:129–135
-
Turner BL, Paphazy MJ, Haygarth PM, McKelvie ID (2002) Inositol phosphates in the surround. Phil Trans R Soc Lond B 357:449–469
-
van Aarle IM, Plassard C (2010) Spatial distribution of phosphatase activity associated with ectomycorrhizal plants is related with soil type. Soil Biol Biochem 42:324–330
-
van Tichelen KK, Colpaert JV (2000) Kinetics of phosphate assimilation by mycorrhizal and non-mycorrhizal Scots pine seedlings. Physiol Plant 110:96–103
-
Vance C, Uhde-Stone C, Allan DL (2003) Phosphorus conquering and use: critical adaptations past plants for securing a nonrenewable resource. New Phytol 157:423–447
Acknowledgements
This review was discussed during the exploratory workshop on "Diversity and Function in Ectomycorrhizal Communities" held in Nancy (December vi–ix, 2009) and funded by European Science Foundation. C. Plassard thanks Dr J. Garbaye (INRA Nancy, France) for his invitation to attend this meeting. The authors thank also 2 anonymous reviewers for their helpful comments.
Open Access
This article is distributed under the terms of the Artistic Commons Attribution Noncommercial License which permits any noncommercial utilize, distribution, and reproduction in any medium, provided the original author(south) and source are credited.
Author information
Affiliations
Corresponding author
Additional information
Handling Editor: Jean Garbaye
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/past-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in whatever medium, provided the original author(s) and source are credited.
Reprints and Permissions
About this article
Cite this article
Plassard, C., Louche, J., Ali, M.A. et al. Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Annals of Forest Science 68, 33–43 (2011). https://doi.org/x.1007/s13595-010-0005-7
-
Received:
-
Accepted:
-
Published:
-
Effect Date:
-
DOI : https://doi.org/10.1007/s13595-010-0005-7
Keywords
- Low-molecular-weight organic acids
- Oxalate
- Phosphatase
- Phytase
- Fungal P transporters
- Phosphate
- Cistron expression
- Organic and mineral phosphorus
Source: https://annforsci.biomedcentral.com/articles/10.1007/s13595-010-0005-7
0 Response to "A Critical Review on the Role of Mycorrhizal Fungi in the Uptake of Phosphorus by Plants"
Post a Comment